Abstract
Introduction
In previously untreated, medically fit patients with chronic lymphocytic leukemia (CLL), the availability of targeted agents has led to research interest in achieving deep, durable remissions and improving survival outcomes. The phase II ICLL-07 (NCT02666898) trial by the French Innovative Leukemia Organization (FILO) evaluated a fixed-duration (15-month) immunochemotherapy approach (no more than 4 fludarabine/cyclophosphamide [FC]-obinutuzumab cycles). Primary analysis showed the approach was safe and 62.2% (84/135) of all patients enrolled achieved complete response (CR) with bone marrow (BM) minimal residual disease (MRD) <0.01% (Michallet et al Lancet Haematol 2019;6:e470-9). Follow-up at median 37 months from treatment start showed a persistent MRD benefit beyond end of treatment, high survival rates, and low long-term toxicity (Michallet et al Blood 2021;137:1019-23). Here we report long-term results at median 64 months from treatment start (data cut-off 20 May 2022).
Methods
Between 27 October 2015 and 16 May 2017, 135 previously untreated, medically fit patients with CLL were enrolled from 27 FILO centers. All received obinutuzumab 1000 mg (8 infusions over 6 cycles) plus ibrutinib (420 mg/day for 9 months). Following evaluation at D1 M9 (N=130 evaluable), those in CR with BM MRD <0.01% then continued only ibrutinib 420 mg/day for 6 additional months (I arm; n=10); otherwise, patients received 4 cycles of FC-obinutuzumab 1000 mg alongside continuing ibrutinib 420 mg/day for 6 additional months (I-FCG arm; n=115). Beyond end of treatment, patients were followed for peripheral blood (PB) MRD every 6 months, response every 3 months, progression-free/overall survival (PFS/OS), and safety. MRD was assessed by 8-color flow cytometry, using a highly sensitive (10-6) technique developed by the FILO group that enables subdivision of classical MRD <0.01% into low-level positive MRD <0.01% and undetectable MRD.
Results
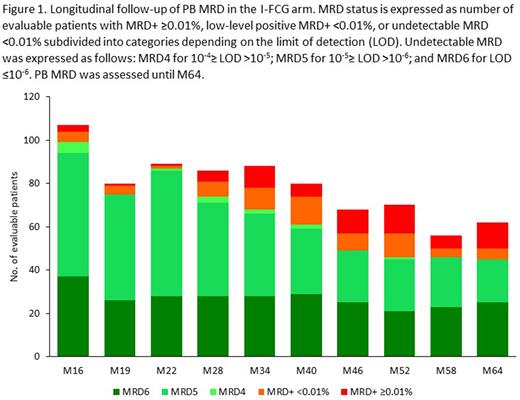
At D1 M16, undetectable PB MRD rates were 80.0% (8/10) in the I arm and 92.5% (99/107) in the I-FCG arm. Beyond end of treatment, in the I arm the undetectable PB MRD rate decreased rapidly, and by M40 all patients had PB MRD positivity (≥0.01% or low-level positive <0.01%). In the I-FCG arm, the undetectable PB MRD rate was 76.3% (61/80) at M40, and of patients currently investigated at M64, 50/62 (80%) still had undetectable PB MRD (Figure 1). Four relapses occurred beyond end of treatment, including 2 CLL progressions (both Richter's syndrome). Throughout follow-up, no differences in undetectable PB MRD rates were apparent in the I-FCG arm according to the IGHV -mutated versus unmutated status (currently at M64: 20/27 versus 25/35, P=0.26). Overall 4-year PFS and OS rates (95% confidence intervals) were 95.5% (92% to 99 %) and 96.2% (93% to 99.5%), respectively. Of 12 deaths overall, 7 occurred beyond end of treatment, with 5 since last publication due to Richter's syndrome at M50, COVID at M50, lung cancer at M50, myelodysplastic syndrome at M60, and acute myeloid leukemia at M69. Other serious adverse events since last publication were metastatic prostate adenocarcinoma at M50 and suspicion of cerebrovascular accident at M65.
Conclusion
Long-term results from the ICLL-07 trial in previously untreated, medically fit patients with CLL demonstrated that that our fixed-duration (15-month) immunochemotherapy approach produced deep and sustained undetectable PB MRD responses that were correlated to PFS, without excess toxicity. These long-term MRD results remain the best to date even compared to the new targeted therapy combinations ie ibrutinib +veneteclax or obinutuzumab + venetoclax A randomized trial is needed to compare our approach with a chemotherapy-free strategy.
Disclosures
Letestu:Abbvie: Consultancy, Other: Funding for congress expenses, Speakers Bureau; AstraZeneca: Speakers Bureau. Le Garff-Tavernier:Abbvie: Consultancy, Honoraria; Janssen: Honoraria. Laribi:AbbVie, AstraZeneca, Beigene, Iqone, Janssen, Novartis, Takeda: Honoraria. Tournilhac:Janssen: Honoraria, Other: Travel grant , Research Funding; Gilead: Honoraria, Other: Travel grant , Research Funding; Securabio: Honoraria, Other: Travel grant , Research Funding; IdeoGen: Honoraria, Other: Travel grant , Research Funding; Abbvie: Honoraria, Other: Travel grant , Research Funding; Takeda: Honoraria, Other: Travel grant , Research Funding. Delmer:Novartis: Honoraria; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Abbvie: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Takeda: Honoraria. Leblond:Abbvie, Beigene, Roche, Amgen, Lilly AstraZeneca, Janssen, Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. de Guibert:AbbVie: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Cartron:Gilead, Novartis, Mylteni, Sanofi, Abbvie, Takeda, Roche, Janssen, Celgene, Novartis, Bristol Myers Squibb: Honoraria; MabQi, Ownards Therapeutics, Abbvie, Roche, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Ysebaert:Abbvie, Astra-Zeneca, Janssen, Roche, Beigene, BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Dartigeas:Janssen, Abbvie, Roche, AstraZeneca: Other: Travel Grant; Abbvie, Roche, Janssen, Beigene, AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Feugier:AstraZeneca, Janssen, Abbvie, Beigene, Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Congress Invitations.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal